pharmastarter
Essentials of Drug Regulatory Affairs in the EU
Welcome!
Guide through the course!
Key concepts
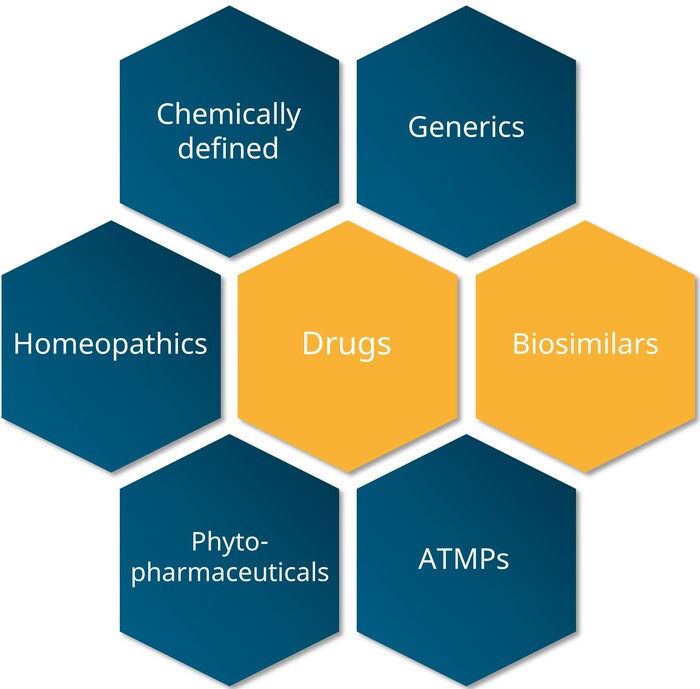
Medicinal products
Development of new medicinal products
Marketing authorisation
Post approval
Pharmacovigilance
Final quiz
The end
Biologicals & Biosimilars

Somatotropin (3D ribbon structure of somatotropin from the PDB, 1HGU).
Somatotropin is the active ingredient of the biological medicinal product "Genotropin" and its biosimilar "Omnitrope", which was authorised in the EU in 2006.
Biologicals & Biosimilars
What are Biologicals?
Biological medicinal products, also known as biologicals, are medicinal products that contain active substances derived from biological sources. Examples of these include proteins from genetically modified mammalian cells.
Active ingredients in biologicals are also characterised by their size and complex molecular structure. In contrast, the active ingredients of chemically synthesised medicinal products are usually much smaller, and the chemical formula of the active ingredient can be clearly described.
Biologicals are usually produced using biotechnology and they are often used to treat oncological diseases.
Examples of biological medicinal products include the following:
Active ingredients in biologicals are also characterised by their size and complex molecular structure. In contrast, the active ingredients of chemically synthesised medicinal products are usually much smaller, and the chemical formula of the active ingredient can be clearly described.
Biologicals are usually produced using biotechnology and they are often used to treat oncological diseases.
Examples of biological medicinal products include the following:
- Insulin
- Vaccines
- Antibodies
- Growth hormones


Somatotropin (3D ribbon structure of somatotropin from the PDB, 1HGU).
Somatotropin is the active ingredient of the biological medicinal product "Genotropin" and its biosimilar "Omnitrope", which was authorised in the EU in 2006.
Biosimilars: similar but not identical
Biosimilars are biological medicinal products that have been developed to be highly similar in all essential aspects (i.e., structure, immunogenicity profile, quality, safety, and efficacy) to an already authorised biological medicinal product. The already authorised biological medicinal product is called the reference product.
Due to their complexity, biosimilars are treated differently from generics in terms of regulatory treatment. As biologics are derived from living organisms, producing identical products using different manufacturing processes is not possible. Proof of bioequivalence is therefore not sufficient for biosimilars. Instead, additional comparability studies must be conducted to show that minor differences do not affect safety and efficacy. For the development of biosimilars, the EMA offers scientific advisory meetings to agree on the studies to be conducted.
Biosimilars, like generics, may only be brought to market after the 10-year marketing protection period of the EU reference product has expired. The first biosimilar in the EU was approved in 2006.
Biosimilars are biological medicinal products that have been developed to be highly similar in all essential aspects (i.e., structure, immunogenicity profile, quality, safety, and efficacy) to an already authorised biological medicinal product. The already authorised biological medicinal product is called the reference product.
Due to their complexity, biosimilars are treated differently from generics in terms of regulatory treatment. As biologics are derived from living organisms, producing identical products using different manufacturing processes is not possible. Proof of bioequivalence is therefore not sufficient for biosimilars. Instead, additional comparability studies must be conducted to show that minor differences do not affect safety and efficacy. For the development of biosimilars, the EMA offers scientific advisory meetings to agree on the studies to be conducted.
Biosimilars, like generics, may only be brought to market after the 10-year marketing protection period of the EU reference product has expired. The first biosimilar in the EU was approved in 2006.